24+ Chapter 9 Review Stoichiometry
Web Chemistry Chapter 9 Stoichiometry Test Review. Web Section 92 Chemical Calculations.
Slideplayer
Web 91 Stoichiometry Basics.

. This is a quick review of some of the sections of chapter 9 of my honors chemistry notes. Web stoichiometry calculations start with a balanced chemical equationThis equation gives the relative numbers of moles of reactants and products. Mole ratios within a chemical reaction.
Web chemistry test chapter 9 review Stoichiometry. Web A common type of stoichiometric relationship is the mole ratio which relates the amounts in moles of any two substances in a chemical reaction. Given the following equation.
Web Quantitative components within a chemical reaction. Click the card to flip. Show all your work in the space provided.
Web Chapter 9 Section 1 Intro to Stoichiometry. Web Modern Chemistry 77 Stoichiometry CHAPTER 9 REVIEW Stoichiometry SECTION 3 PROBLEMS Write the answer on the line to the left. Chapter 9 Complete Stoichiometry Review Practice.
Web 15 mol O2 x 2 mol KClO3 10 mol KClO3. Stoichiometry analysis of a chemical reaction. Quantitative components within a chemical reaction.
Click the card to flip. -the law of conservation of mass. Stoichiometry is the branch of chemistry that deals with elements in compounds and with reactants and products in chemical.
What pollutant forms when automobile emissions react with oxygen gas and ultraviolet rays. We can write a mole ratio for a. Web From a general summary to chapter summaries to explanations of famous quotes the SparkNotes Review of Stoichiometry Study Guide has everything you need to ace.
Use balanced chemical equations to derive stoichiometric factors relating amounts of reactants and. Terms in this set 13 A balanced chemical equation plays an important role in stoichiometric calculations because. 443 views 4 years ago Chem in 15 minutes or Less.
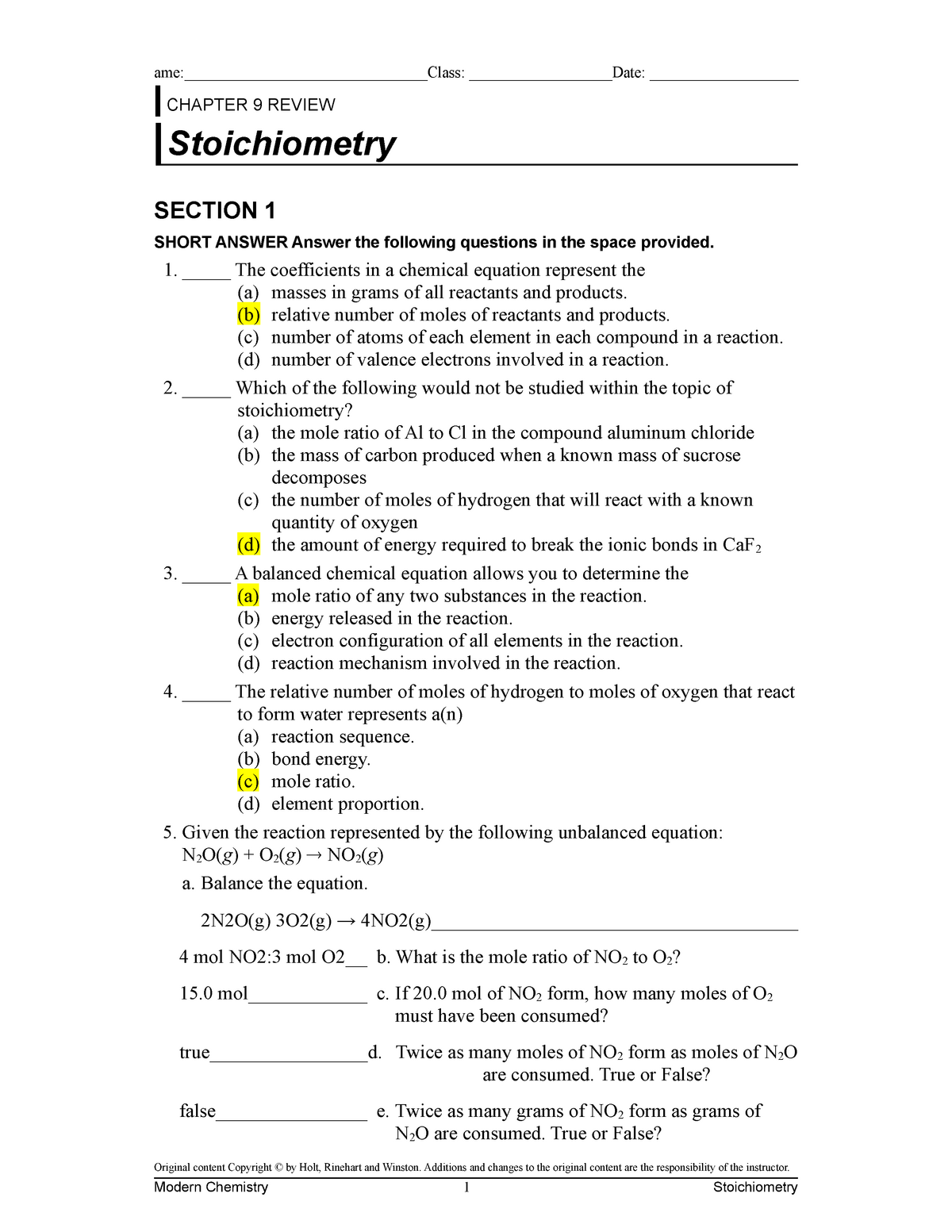
Explain the concept of mole ratio as used in reaction stoichiometry problems. Web CHAPTER 9 REVIEW Stoichiometry MIXED REVIEW SHORT ANSWER Answer the following questions in the space provided. 6 NaBr Mg3PO42 2 Na3PO4 3 MgBr2.
Stoichiometry analysis of a chemical reaction involving moles and mass. Web In this chapter you will learn about. What is the source of this ratio.
C 3H 4g. Stoichiometry Review and Chapter Summary. N 2g 3H 2g 2NH 3 g.
Use the above balanced. A balanced chemical equation. The number of moles and the mass of chlorine Cl 2 required to react with 100 g of sodium metal Na.
The efficiency of a reaction is measured by the. Show all your work in the space. Web Explain the concept of stoichiometry as it pertains to chemical reactions.
Mole ratios within a chemical reaction. Chapter 9 quiz 1- mole to mole calculations. And 92 Mole-Mass and Mass-Mass Calculations.
In this section we will review some of the ideas related to stoichiometry that we have previously seen earlier in this textbook. Web SECTION 9-1 continued PROBLEMS Write the answer on the line to the left. Given the following equation.
Web Chapter 9 Combustion of Phosphorus and Limiting Reactantpdf. Construct mole ratios from balanced chemical equations and apply these ratios in mole-mole stoichiometric calculations.
Slideserve

Scribd

Yumpu

1

Acs Publications American Chemical Society

Sciencedirect Com
Slideplayer

Slideplayer

Course Hero
Studocu

Quizlet

Course Hero

Slideplayer
1

Course Hero

2

Slideshare